/ Ronny Hardegger
New Insights into Sustainable Photochemistry: Cr, Co, and Fe in the Spotlight



The research team led by Professor Oliver S. Wenger at the University of Basel has published three new articles in the "Journal of the American Chemical Society" that deepen our understanding of the photophysics and photochemistry of first-row transition-metal complexes. Focusing on chromium (Cr), cobalt (Co), and iron (Fe) systems, these studies deliver new design principles, uncover unexpected structure–property relationships, and correct calculation errors in predicting light-driven reactivity. Such insights support the shift toward more sustainable photochemistry using earth-abundant metals instead of costly precious metals like ruthenium and iridium.
Expanding Chromium’s Role in Photochemistry
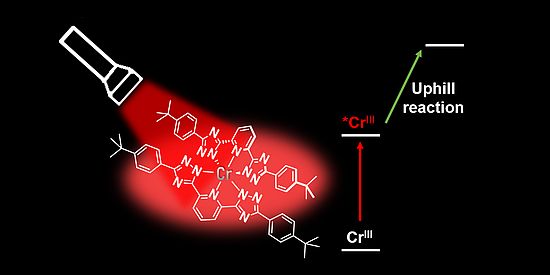
Giacomo Morselli and colleagues have developed a new class of chromium(III) complexes featuring exceptionally long-lived excited states, if compared to conventional metal complexes. These allow the complexes to accept electrons from a broad range of donors, even when the electron transfer is energetically uphill. The resulting reduced chromium species have enhanced reducing power thanks to a new ligand design that increases metal–ligand bond covalency. Powered by low-energy red light, the system expands chromium’s role beyond its traditional use in oxidative photochemistry through thoughtful ligand design, offering a promising advance for more sustainable and energy-efficient photocatalysis.
Surprising Lessons from Cobalt Photochemistry
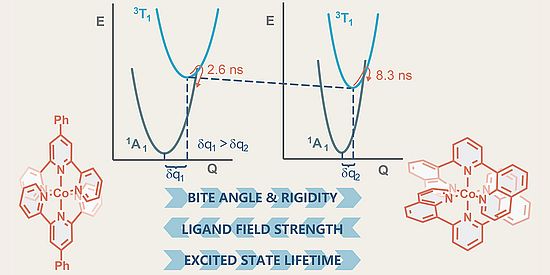
Polina Yaltseva and colleagues have uncovered unexpected behavior in cobalt(III) complexes that challenges design principles established for chromium(III). In CrIII polypyridine systems, increasing the bite angle (the angle at the metal center between the two binding points of a chelating ligand) strengthens the ligand field by enhancing metal-ligand orbital overlap. This increased overlap raises the energy of metal-centered excited states through greater π-backbonding from pyridine ligands, thereby extending the excited-state lifetimes. In CoIII systems, however, the trend is reversed. Here, these ligands act predominantly as π-donors rather than π-acceptors, and larger bite angles weaken the ligand field. Counterintuitively, the resulting lower-energy excited states can persist longer than expected, due to π–π stacking interactions between ligands that rigidify the structure and suppress nonradiative decay. This unusual interplay of electronic and structural effects opens new strategies for the design of sustainable, cobalt-based photocatalysts.
A Reality Check for Iron Catalysts
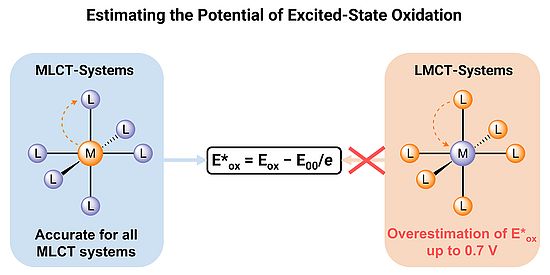
Joël Wellauer and colleagues have found that the widely used method for estimating the excited-state redox potentials of transition-metal complexes can give misleading results in the case of iron(III) with ligand-to-metal charge-transfer (LMCT) excited states. Light absorption normally increases the reactivity of a complex, making it either a stronger oxidant (accepting electrons) or a stronger reductant (donating electrons). Chemists therefore want to quantify how much the redox potential shifts upon excitation, since this determines what reactions the complex can undergo. Normally, excited-state redox potentials are estimated from the ground-state redox potential, adding the excitation energy for reductions or subtracting it for oxidations. This works well for reductions and gives reliable values for Fe(III) LMCT complexes. For oxidations, however, it can be off by as much as 0.7 volts. The problem is that light absorption changes the electronic configuration: in the ground state, oxidation removes an electron from the metal, while in the LMCT excited state the missing electron (the ‘hole’) is located on the ligand. Since these oxidized forms are electronically distinct, the simple formula breaks down. This correction is crucial for reliably designing photooxidations with Fe(III) and other earth-abundant metals such as chromium and manganese.
Together, the three studies provide new insights into how first-row transition-metal complexes behave under light. By revealing new design principles, unexpected behaviors, and critical corrections, they push the field closer to replacing scarce precious metals with abundant, sustainable alternatives and hence laying the groundwork for the next generation of photocatalysts.
Original Publications
Giacomo Morselli, Tim H. Eggenweiler, Marco Villa, Alessandro Prescimone, Oliver S. Wenger
Pushing the Thermodynamic and Kinetic Limits of Near-Infrared Emissive CrIII Complexes in Photocatalysis
J. Am. Chem. Soc.2025, 147, 31, 28226–28240, doi: 10.1021/jacs.5c08541
Polina Yaltseva, Tamar Maisuradze, Alessandro Prescimone, Stephan Kupfer, Oliver S. Wenger
Structural Control of Metal-Centered Excited States in Cobalt(III) Complexes via Bite Angle and π–π Interactions J. Am. Chem. Soc.2025, 147, 32, 29444–29456, doi: 10.1021/jacs.5c08841
Joël Wellauer, Michael L. Pattuwage, Egan H. Doeven, Timothy U. Connell, Oliver S. Wenger, and Paul S. Francis
Rethinking the Excited-State Redox Properties of Iron(III) Complexes for LMCT Photoredox Catalysis
J. Am. Chem. Soc.2025, 147, 32, 29304–29314, doi: 10.1021/jacs.5c08841
Further Information
Website research group Prof. Oliver Wenger
Quick Links