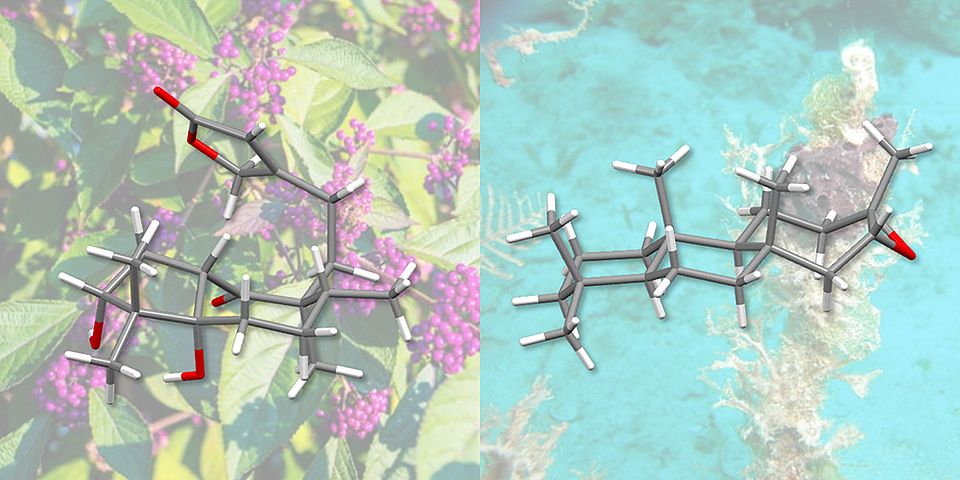New approach in the synthesis of complex natural substances
They are found as fragrances in cosmetics or as flavorings in food, and form the basis of new medications: Terpenes are natural substances that occur in plants, insects and sea sponges. They are difficult to produce synthetically. However, chemists at the University of Basel are now introducing a new method of synthesis.
Many natural substances possess interesting characteristics, and can form the basis of new active compounds in medicine. Terpenes, for example, are a group of substances, some of which are already used in therapies against cancer, malaria or epilepsy.
There is an important prerequisite for their development into drugs, however: these terpenes should be produced synthetically from simple starting materials. This allows their exact molecular structure to be controlled, and targeted changes to be made to improve their properties.
New approach for total synthesis
Professor Olivier Baudoin and his doctoral student Oleksandr Vyhivskyi have now developed a new approach to the total synthesis of these substances and have used it to artificially produce two diterpenes – a subclass of terpenes: randainin D and barekoxide. Their report appears in the Journal of the American Chemical Society.
Randainin D, originally extracted from plants, inhibits production of an enzyme that plays a role in conditions such as rheumatoid arthritis, cystic fibrosis and chronic obstructive pulmonary disease. It might therefore be considered as a potential candidate for the development of new therapeutic agents.
The chemists have managed to synthesize randainin D in 17 steps. To this purpose, they employed both a chemical reaction called ring-closing metathesis and photocatalysis – a process in which chemical reactions are promoted by light energy.
Construction of complex structures
In this way, they succeeded in creating the complex ring structure of the molecule, as well as inserting a chemical building block called an allyl group, made up of three carbon atoms. An allyl group is a useful component in the synthesis of various organic substances, as it serves as a reaction partner during the construction of the desired structure.
Finally, the same synthesis method was used by the researchers to produce barekoxide. Although barekoxide had already been produced synthetically by another research group in 2010, Baudoin and Vyhivskyi’s approach has reduced the number of steps from ten to seven, thus significantly simplifying the total synthesis of this compound.
“Our results show the potential of photocatalysis for the total synthesis of complex terpenes. This could pave the way for the development of new medications,” concludes Baudoin.
Original Publication
Oleksandr Vyhivskyi, Olivier Baudoin
Total Synthesis of the Diterpenes (+)-Randainin D and (+)- Barekoxide via Photoredox-Catalyzed Deoxygenative Allylation
Journal of the American Chemical Society, doi: 10.1021/jacs.4c02224
Further Information
Website research group Baudoin
Quick Links