Stable membrane for therapeutic carriers
Cells can generate vesicles as a response to changes in their environment. Although such cell-derived vesicles have great potential for biomedical research, their membrane is fragile and they have tendency to cluster together. Researchers at the University of Basel have successfully introduced a strategy to overcome these issues by equipping the vesicular membrane with a stabilizing shell.
Cell-derived vesicles are small, bubble-like vessels that the cell generates out of its own membrane and cytoplasm when it is stressed by radiation or certain chemicals. These vesicles consist of an outer membrane and a hollow cavity in which they can transport a variety of biomolecules. They are structurally very similar to their mother cell, but can exist independently of it. Both their function as extracellular delivery vehicles and their similarity to the original cell make them attractive for the development of advanced therapeutic carriers.
However, all cell-derived vesicles tend to rupture and aggregate. Their membrane is very unstable and they cannot survive long inside an organism. This severely limits their possible applications in medical and pharmaceutical research.
An elegant solution
Using a new approach, a research team led by Prof. Dr. Cornelia Palivan from Department of Chemistry at the University of Basel and the National Center of Competence in Research Molecular Systems Engineering was able to both prevent the deterioration of the membrane and avoid the formation of clusters in a specific type of cell-derived vesicle. Their findings were published in the scientific journal Advanced Healthcare Materials.
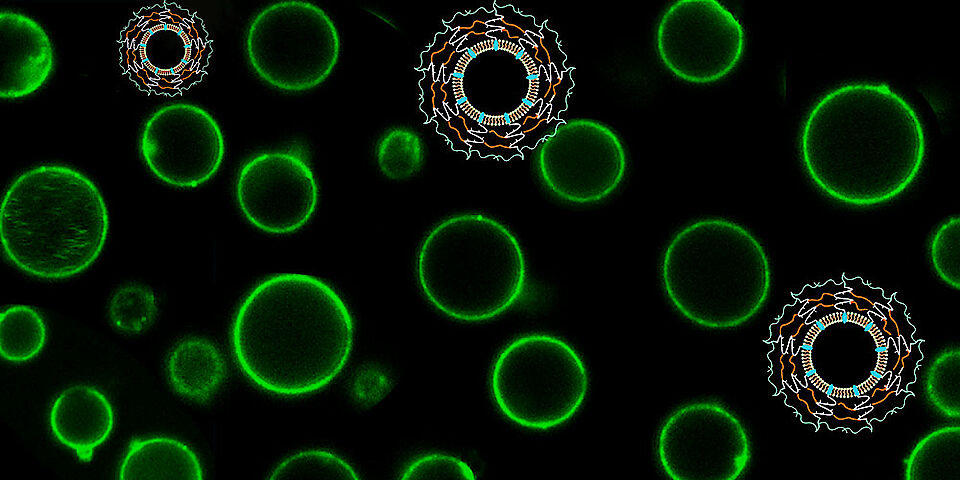
The researchers achieved this by decorating the membrane of giant plasma membrane vesicles (GPMVs) with a synthetic copolymer that acts as a protective layer. “We took another view on improving the stability and functionality of natural membranes of cell-derived vesicles as required for advanced medical applications,” explains Palivan.
The chemists synthesized the copolymer to specifically contain three domains: the first is a hydrophobic domain which attaches itself to the vesicle membrane and serves as an anchor. A second, hydrophilic domain forms an external shell by means of chemical cross-linking. This reaction induces the formation of the protective shell which increases the stability of the membrane while still allowing the GPMV to carry out its function as a transport or storage vessel. A third domain enhances the overall biocompatibility.
The cross-linked GPMV exhibited reduced membrane permeability, increased resistance against surfactants and various storage temperatures, as it was shown in a collaboration with researchers at the ETH Zurich. There was also no aggregation in time.
Controlled release of cargo
In addition to stabilizing the membrane of the GPMV, the researchers demonstrated that they now also had the ability to control loading and releasing of the cargo. The vesicles shrink or swell depending on changes of the pH in their environment. When they swell, they can release a specific cargo already encapsulated inside, such a drug. Therefore, they act as stimuli-responsive therapeutic carriers that release the drug "on demand", when there is a defined change in the pH of their environment.
Applications in cancer therapy
“Our approach brings up the first example of a copolymer-stabilized GPMV capable to trigger on-demand permeation of molecules,” says Xinan Huang, the first author of the study. This new property of stimuli-responsiveness induced by the chemical nature of the copolymer makes these modified transport vesicles of great interest for biomedical research. This is particularly the case for cancer therapy, since the tumor microenvironment displays a lower pH than that of healthy tissues. It is possible to load the GPMVs with medication which then gets delivered directly into the target cell.
Although the results are currently limited to one type of vesicle, the research group plans to expand their research even further: “This strategy is so straightforward that it should be easily scaled-up with significant efficiency and extended to other cell-derived vesicles,“ Cornelia Palivan says.
Original publication
X. Huang, D. Hürlimann, H. T. Spanke, D. Wu, M. Skowicki, I. A. Dinu, E. R. Dufresne, C. G. Palivan
Cell-derived Vesicles with Increased Stability and On-Demand Functionality by Equipping their Membrane with a Cross-linkable Copolymer
Advanced Healthcare Materials (2022), DOI:10.1002/adhm.202202100.
Further Information
Website Palivan research group
Quick Links