Research Topics
My research is concerned with enzymes that catalyze unusual reactions. These investigations involve the purification of unknown enzymes, the study of their respective mechanisms and the synthesis of synthetic enzyme models. The focus on active site analogues evolved because catalytic systems can be created displaying reactivity comparable to the enzymes, and intermediates of the reaction cycles can be synthesized which have escaped detection so far. Provided these synthetic intermediates are structurally well characterized and kinetically competent this wisdom is likely to be useful to identify the corresponding intermediates at the protein. This research at the interface of organic chemistry, biochemistry, and inorganic chemistry finally provides information which often can not be obtained from investigating the proteins itself and hence is a step forward to understand how nature catalyzes sophisticated reactions.
Research Topics
Bioorganic Chemistry
- Enantioselective/diastereoselective protonation of double bonds
- Synthesis of new metal complexes for biomimetic catalysis
2.1. Enantioselective reduction of aliphatic ketones
2.2. Oxidation of non-activated positions
2.3. Regioselective double bond cleavage of carotenoids - Drug-drug interaction - synthesis of inhibitors of cytochrome P450 3A4
1. Enantioselective/diastereoselective protonation of double bonds
We discovered the enzyme tocopherol cyclase in the cyanobacteria Anabaena variabilis, subsequently determined its substrate specificity and recently cloned the protein from the related Anabaena sp. into E. coli. The enzymatic reaction mechanism was determined by isotopic labeling experiments which revealed that the reaction proceeds by si protonation of the double bond of 1 followed by re attack of the phenolic oxygen to yield γ-tocopherol 2 suggesting a concerted, nonsynchronous cyclization, during which positive charge develops at the carbon atom (see 3) that is trapped by the phenol.[1]

Recently we developed a synthetic procedure which mimics this enzymatic reaction.[1] A chiral dipeptide handle was used, see 4, that induces a diastereoselective protonation/cyclization with up to 80%de. The product of the ring closure can be easily converted into α-tocopherol 5, the biologically most important member of the vitamin E family.

2. Synthesis of new metal complexes for biomimetic catalysis
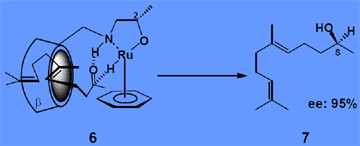
2.1. Enantioselective reduction of aliphatic ketones
Attaching metal complexes to cyclodextrins conceptually generates enzyme-like catalysts containing a hydrophobic substrate binding site and a centre of reactivity, see 6. The supramolecular system allows for hydrogen transfer reactions (HCOONa, water) to non conjugated ketones such as geranylacetone yielding the corresponding alcohol 7 with excellent ee.[2]
2.2. Oxidation of non-activated positions
Heme thiolate proteins - in particular cytochromes P450 - are the biological paradigma for catalysts which insert oxygen into more or less activated C-H bonds (benzylic, aromatic, -CH3), epoxidize double bonds and perform oxidations at or adjacent to heteroatoms. All cytochromes P450 contain the same cofactor: an iron protoporphyrin (IX) with a thiolate ligand coordinating to iron, see 8. Synthetic enzyme models have contributed a great deal to understand the complicated catalytic cycle of oxygen activation, reductive oxygen cleavage and oxygen insertion (monooxygenase).[3] From these studies and investigations of cytosolic P450 enzymes the high-valent O=Fe(IV) porphyrin radical cation 9 emerged as the most reactive candidate for oxygen insertion.

Since in model systems both the thiolate ligand and the porpyhrin chromophore are rather sensitive in the presence of excessive co-oxidant we recently established new P450-like systems that contain a SO3- ligand coordinating to iron. Complexes such as 10 indeed show features similar to P450 with respect to E0, spectroscopy and reactivity[4],[5] and moreover equivalents of 9 can be generated and characterized.[6] Due to its relative stability systems such as 10 have a great potential for application in metabolism research.

2.3. Regioselective double bond cleavage of carotenoids
The oxidative cleavage β-carotene 11 is an important enzymatic reaction providing retinal 12 (provitamin A) for vision. We discovered this enzyme in chicken's intestinal mucosa, overexpressed the protein in E. coli and BHK cells,[7] we determined its substrate specificity and the reaction mechanism.[8] These investigations led to the conclusion that the central cleavage of 11 is catalyzed by non-heme iron monooxygenase.[9]
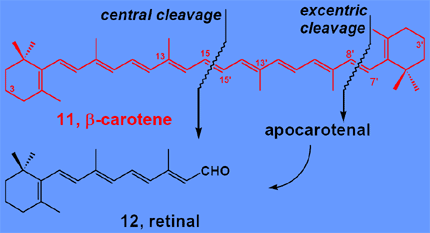
Two supramolecular systems, 13[10] and 14, were synthesized and were shown to catalyze the central cleavage of carotenoids selectively.

3. Drug-drug interaction - synthesis of inhibitors of cytochrome P450 3A4
Cytochrome P450 3A4 (CYP 3A4), a heme-thiolate protein, is one of the most important P450s in human liver. The ability of CYP 3A4 to metabolize >50% of administered therapeutic agents accounts for the large number of documented drug-drug interactions associated with CYP 3A4 inhibition. It is therefore of great importance to know as early as possible the affinity of new drug candidates to CYP 3A4, in order to determine their potential as inhibitors, and to evaluate their influence on the metabolism of co-administered drugs. Some particular features of CYP 3A4 make it difficult to achieve this aim. Several distinct binding sites have been described for prototype substrates of CYP 3A4. Further it has been shown that more than one substrate molecule can be accommodated by the active site. Partial inhibition and even activation have been observed when pair of drugs was co-incubated.

We have designed and synthesized several steroid derivatives, e.g. 15,[11] which display IC50 values between 1-10μM for various CYP 3A4 substrates, which is considered to be a cut-off value to eliminate drugs binding too strongly to CYP 3A4. Further, the probe is compatible with high-throughput technology due to the presence of the chromophore in 15 whose fluorescence is quenched by the iron porphyrin on binding to the active site. Thus the affinity of drug candidates can be rapidly evaluated by displacement of a general inhibitor such as 15 without performing tedious enzyme inhibition studies.
1.
A Biomimetic Cyclization Leading to α-Tocopherol
C. Grütter, E. Alonso, A. Chougnet, W.-D. Woggon
Angew. Chem. Int. Ed. 2006, 45, 1126.
2.
Enantioselective Reduction of Aromatic and Aliphatic Ketones Catalyzed by Ruthenium Complexes Attached to β-Cyclodextrin
A. Schlatter, M. K. Kundu, W.-D. Woggon
Angew. Chem. Int. Ed. 2004, 43, 6731.
3.
Metalloporphyrines as Active Site Analogs - Lessons from Enzymes and Enzyme Models
W.-D. Woggon
Acc. Chem. Res. 2005, 127.
4.
Reactivity of a New Class of P450 Enzyme Models
D. Meyer, T. Leifels, L. Sbaragli, W.-D. Woggon
Biochem. Biophys. Res. Commun. 2005, 338, 372.
5.
Synthetic Models of Cytochrome P450 Enzyme: How Different Are They from the Natural Species?
S. Kozuch, T. Leifels, D. Meyer, L. Sbaragli, S. Shaik, W.-D. Woggon
Synlett (Cluster) 2005, 765.
6.
The Complete Catalytic Cycle of a New Enzyme Mimic of Cytochrome P450
A. Franke, N. Hessenauer-Ilicheva, D.Meyer, W.-D. Woggon, R. van Eldik
Journal of the American Chemical Society2006, 128 (41), 13611-13624 .
7.
Cloning and Expression of β,β-Carotene 15,15'-Doxygenase
A. Wyss, G. Wirtz, W.-D. Woggon, R. Brugger, M. Wyss, A. Friedlein, H. Bachmann, W. Hunziker
Biochem. Biophys. Res. Com. 2000, 271, 334.
8.
The Reaction Mechanism of the Enzyme-Catalyzed Central Cleavage of β,β-Carotene to Retinal
M. G. Leuenberger, C. Engeloch-Jarret, W.-D. Woggon
Angew. Chem. Int. Ed. 2001, 2613.
9.
Oxidative Celavage of Carotenoids by Enzyme Models and β-Carotene 15,15'-monooxygenase
W.-D. Woggon
Pure & Appl. Chem. 2002, 74, 1397.
10.
A Supramolecular Enzyme Mimic That Catalyzes the 15,15' Double Bond Scission of β,β-Carotene
R. R. French, P. Holzer, M. Leuenberger, W.-D. Woggon
Angew. Chem. Int. Ed. 2000, 39, 1267.
11.
Design and Synthesis of a New Fluorescent Probe for Cytochrome P450 3A4
A. Chougnet, C. Stoessel, W.-D. Woggon
Bioorg. Med. Chem. Letters 2003, 13, 3643-3645.
Quick Links
