/ Research
Paramagnetic lanthanoids as key tools to determine high-energy states of proteins
University of Basel researchers have achieved unprecedented insight into the paramagnetism of lanthanoid (III) complexes. This class of compounds was then used in a collaboration with a research team from Brandeis University (MA, USA) to characterise for the first time short-lived, sparsely populated, high-energy states of proteins by NMR. The surprising details of the structure and the free energy landscape of an enzyme open very promising approaches for future applications of this new method.
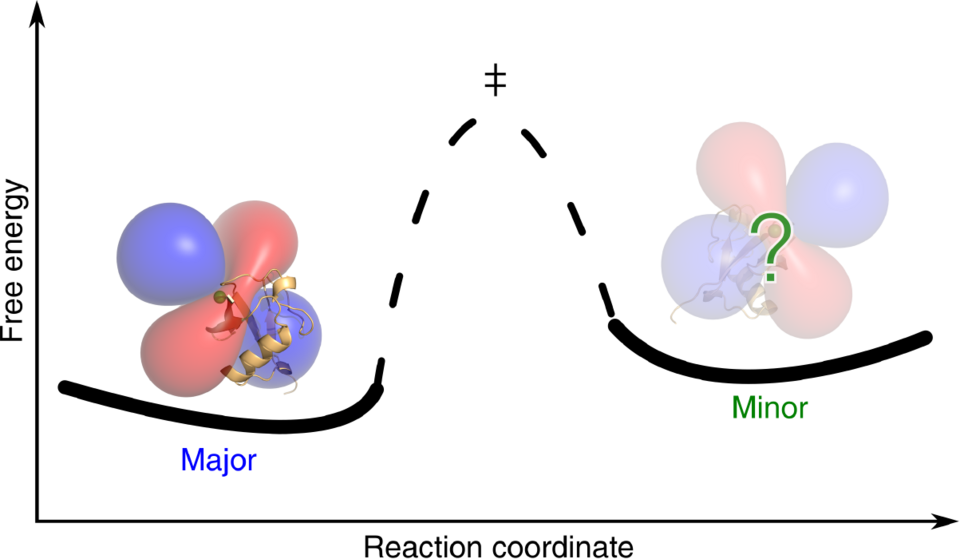
Proteins are often represented by a single, static structure that can be determined by a variety of methods. It is, however, known for a long time, that the conformational landscape of proteins is much more complex, and that the dynamic nature of these molecules is responsible for their unique properties.
The Häussinger group designs and synthesises since almost 20 years so-called lanthanide chelating tags (LCTs). In LCTs a cage-like molecule encapsulates the lanthanoid and a cysteine reactive activator allows to permanently connect the LCT to a single cysteine mutant of the desired protein. The resulting changes in the NMR spectra allow to determine structure, dynamics and interactions of the protein. The “anisotropic paramagnetic susceptibility tensor” (χ-tensor) describes the paramagnetic properties of the LCT and can be calculated from the induced chemical shift changes of the proteins NMR spectra.
These shift changes are termed “pseudocontact shifts” (PCSs), depend on the χ-tensor and are the prerequisite for any analysis of the protein properties of interest. However, any motion of the LCT relative to the protein reduces the PCSs as well as the apparent χ-tensor due to motional averaging.
How to outsmart the motion of molecules
Recently Häussinger’s PhD co-workers Raphael Vogel and Thomas Müntener achieved a breakthrough in the understanding of the complex way in which the PCSs are induced in proteins by LCTs. In order to overcome the motional averaging, Vogel stood up to the challenge to assign every single proton within the LCT itself – a formidable task in a molecule with such a strong paramagnetic centre, where modern multidimensional NMR methods cannot be applied. He combined epic synthetic efforts to deuterate site-specific positions with a clever combinatorial approach to achieve the desired assignment for the entire series of lanthanoids. In addition to the gain of unprecedented knowledge of the “intrinsic” χ-tensors, the researchers also observed a characteristic modulation of the magnetic properties as a function of the lanthanoid metal used – this is the first evidence that the so-called ligand field effect is also relevant for this class of metals and this will have consequences for the design and application of future LCTs as well as for single molecule magnets.
A new NMR technique allows to understand high-energy states of proteins
Furthermore, this detailed understanding helps tremendously to select the optimal LCT for a specific task. As a direct consequence of the extended insight into tag movement, a successful collaboration with the research group of Prof. Dorothee Kern at Brandeis University could be established. Kern’s team investigates the conformational space that proteins cover and focuses on short-lived, high-energy states that can be adopted by these molecules. In fact, it is often required that such a high-energy conformation is populated to perform a certain reaction, e.g. in an enzyme. The ultimate challenge to structurally characterise these sparsely populated, high-energy states in atomistic detail has now been achieved for the first time by combining a specific NMR technique called Carr-Purcell-Meiboom-Gill (CPMG) relaxation dispersion with PCSs. The new technique, termed PCS-CPMG, was applied to three metal-binding proteins, in which the paramagnetic metal binds directly to the enzyme. In order to exploit PCS-CPMG for all proteins of interest, LCTs have to be used to introduce lanthanoids to non-metal binding proteins. Careful selection of the LCT with respect to the motion relative to the protein and a well-considered choice of the lanthanoid metal allowed Pascal Rieder from the Häussinger group for the first time the successful application of PCS-CPMG to the proteins ubiquitin and the dimeric chaperone trigger factor (TF). For both proteins, movements of loops or entire domains in high-energy states could be elucidated using the new technique leading to a more detailed understanding of their mode of action.
Future applications of PCS-CPMG will pave the way to a much more detailed understanding of the mechanisms of enzyme chemistry which is relevant for fundamental research as well as for medical implications like the design of new pharmaceuticals.
Original publications
John B. Stiller, Renee Otten, Daniel Häussinger, Pascal S. Rieder, Douglas L. Theobald, Dorothee Kern
Structure determination of high-energy states in a dynamic protein ensemble
Nature 603/7901 (2022) 528-535. doi: 10.1038/s41586-022-04468-9
Raphael Vogel, Thomas Müntener, Daniel Häussinger
Intrinsic anisotropy parameters of a series of lanthanoid complexes deliver new insights into the structure-magnetism relationship
Chem 7/11, (2021), 3144-3156. doi: 10.1016/j.chempr.2021.08.011
Further infromation
Research group of Prof. Häussinger
Quick Links