Research Topics
1. Matrix Isolation
2. Ion Trap
1. Matrix Isolation
Our oldest and most versatile setup employs a combination of mass selection with matrix isolation spectroscopy. In these experiments reactive molecular ions are produced in an appropriate source, guided through a mass-selective lens system and frozen in a noble gas matrix at low temperatures. Electronic absorption spectra of many different species can be studied, from small clusters such as B3 and Si4 to long linear carbon chains like C17 and HC15H. The main limitation in matrix isolation spectroscopy are the matrix effects, namely that noble gas atoms have a perturbing influence on the species they surround. In absorption spectroscopy this leads to shifts and line broadening, making comparison between laboratory and astronomical observations difficult. However, matrix data provide a good starting point for high-resolution, gas-phase measurements.
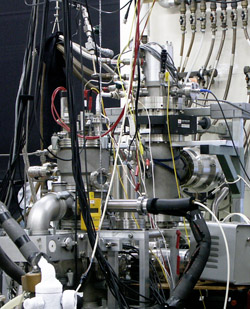
Below is a schematic of the experiment. Generated ions are extracted from the source and guided through a differentially pumped vacuum system involving electrostatic lenses, a 90o deflector and a quadrupole mass filter. Ions with a chosen m/z are deposited with excess of neon onto a rhodium-coated sapphire plate that is mounted on a copper piece, which is cooled by a closed-cycle helium cryostat to temperatures around 6 K. To obtain detectable concentration of the ions, several hours of deposition is required. The detection system for the recording of electronic spectra consists of a broadband light source and a spectrograph, equipped with three rotatable gratings and two range-specific CCD cameras. Besides ions, neutrals may also be investigated after generation by a standard photobleaching technique. A custom-modified IR spectrometer complements the UV/Vis measurements.

Publications
Electronic Transitions of C5H3+ and C5H3 : Neon Matrix and CASPT2 Studies
Jan Fulara, Arghya Chakraborty, Adam Nagy, Karol Filipkowski, and John P. Maier
J. Phys. Chem. A 2015, 119, 11, 2338-2343
Spectroscopic characterization of C7H3+ and C7H3•: electronic absorption and fluorescence in 6 K neon matrices
Arghya Chakraborty, Jan Fulara, Rainer Dietsche and John P. Maier
Phys. Chem. Chem. Phys. 2014, 16, 7023-7030
Electronic Transitions of C6H4+ Isomers: Neon Matrix and Theoretical Studies
Jan Fulara, Adam Nagy, Karol Filipkowski, Venkatesan S. Thimmakondu; John F. Stanton, John P. Maier
J. Phys. Chem. A 2013, 117(50), 13605-13615
Electronic Spectroscopy of a C7H4+ Isomer in a Neon Matrix: Methyltriacetylene Cation
Arghya Chakraborty, Jan Fulara, John Maier
Aust. J. Chem. 2013, 67(3), 416-419
2. Ion Trap
Producing cold molecules becomes more difficult as the size of the species increases. This is due to the fact that larger molecules have more internal vibrations where the energy can be stored. Therefore very big molecules, like polycyclic aromatic hydrocarbons (PAHs) and fullerenes, cannot be cooled efficiently during the short-time scales involved in a supersonic expansion. An alternative approach is to collect the ions in an ion trap and cool them down through collisions with a cold buffer gas, like helium or argon.

Publications
(1)1A'- X1A' Electronic Transition of Protonated Coronene at 15 K
C. A. Rice , F.-X. Hardy , O. Gause , and J. P. Maier
J. Phys. Chem. Lett. 2014, 5(6), 942-945
Absorptions in the Visible of Protonated Pyrene Collisionally Cooled to 15 K
F.-X. Hardy, O. Gause, C. A. Rice, and J. P. Maier
ApJL 2013, 778 (2), L30
A Novel Method to Measure Electronic Spectra of Cold Molecular Ions
Satrajit Chakrabarty, Mathias Holz, Ewen Kyle Campbell, Agniva Banerjee, Dieter Gerlich, and John Paul Maier
J. Phys. Chem. Lett. 2013 4, 4051-5054
Electronic Absorption Spectrum of Triacetylene Cation for Astronomical Considerations
S. Chakrabarty, C.A. Rice, F. J. Mazzotti, R. Dietsche & J.P. Maier
J. Phys. Chem. A 2013, 117 (39), 9574-9577
Quick Links
